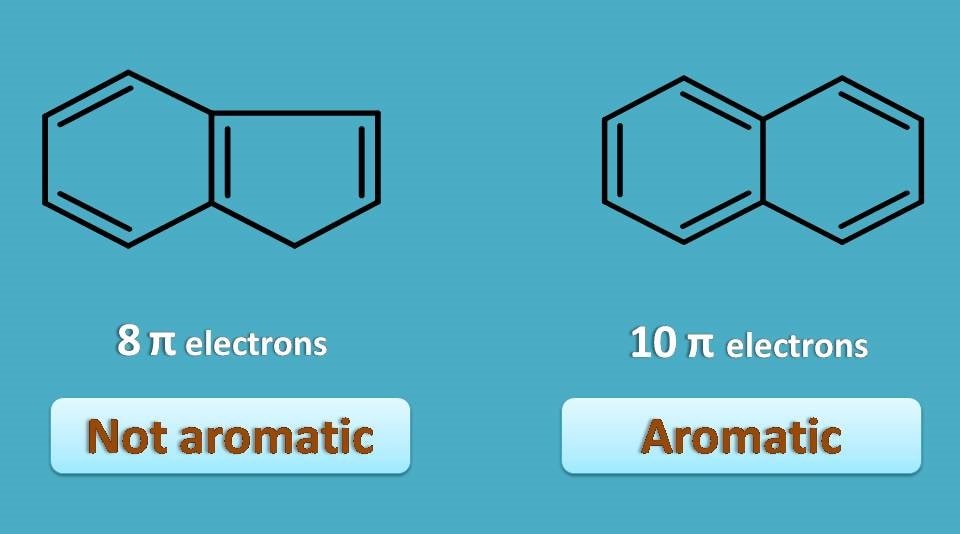
Aromaticity 4 Criteria Every Compound Needs
Anti Aromatic compounds are those compounds that satisfy the rules of planarity and fully conjugation of the pi electrons inside the ring but fail to satisfy Huckel's rule of 4n2 pi electrons The antiaromatic compound contains 4nπ electronsOption C, is a NonAromatic Compound as it does not satisfy the condition of Planarity Huckel's Rule 4n2 = Number of Resonating Electrons Resonating electrons include both pi electrons and lone pairs When solving for 'n', n must equal to a whole number If we get a fraction then the molecule DOES NOT obey Huckel's rule Benzene is the most common aromatic molecule a benzene ring has 3 pi bonds thus 6 resonating
